Delayed Sleep Phase Syndrome (DSPS) in Adolescents: Causes, Treatment, and Light Therapy Innovations

Delayed Sleep Phase Syndrome (DSPS)
Delayed Sleep Phase Syndrome , also known as Delayed Sleep-Wake Phase Disorder, is a chronic disruption of the body’s sleep-wake cycle. In DSPS, a person’s natural sleep period is shifted much later than what is socially or medically considered normal. In practical terms, someone with DSPS (often nicknamed a “night owl”) cannot fall asleep until very late at night (often a phase delay of 2–6 hours) and struggles to wake up in time for typical morning obligations like school or work. This condition is the most common type of circadian rhythm disorder, meaning it involves a misalignment of one’s internal clock with the external day-night schedule.
How common is DSPS?
It is especially prevalent in adolescents and young adults. Roughly 7–16% of adolescents are estimated to meet criteria for DSPS, making it a significant issue for high school and college-age populations. The onset can occur in early childhood, but it most commonly emerges around puberty and the teenage years. During adolescence, biological changes in circadian timing occur: the brain’s biological clock (the circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus) naturally shifts later. Puberty triggers a delayed timing of melatonin release by about 1–3 hours, meaning teens start feeling sleepy much later in the evening. This dim light melatonin onset (DLMO) shift is a normal part of development, but in DSPS it can be exaggerated. Furthermore, teenagers often exhibit an evening chronotype—a preference for staying up late—due to these hormonal shifts and increased independence. They may not feel sleepy until very late at night, yet still require early wake times, leading to chronic misalignment.

Why does DSPS happen?
The causes of DSPS are multifactorial, involving a mix of biological and social influences:
Biological factors:
DSPS stems partly from a longer intrinsic circadian rhythm (>24 hours), causing the internal clock to run late. Genetic mutations, such as in the CRY1 or PER3 genes, are linked to familial DSPS. Twin studies show 40–50% heritability for sleep timing. Adolescents may have increased sensitivity to evening light, with normal indoor lighting or screens delaying melatonin and shifting sleep timing. One study found that light exposure significantly suppressed melatonin in pubertal teens, emphasizing the impact of nighttime screen use.
Psychosocial and behavioral factors:
Late-night use of smartphones, computers, or gaming exposes teens to blue light, delaying sleep onset. Irregular sleep patterns (weekend sleep-ins) cause social jetlag. Evening commitments—jobs, homework, or socializing—and reduced parental oversight contribute to inconsistent schedules. These behaviors reinforce circadian delay, creating a mismatch between the teen’s internal clock and societal demands.
What are the consequences?
When allowed to follow a delayed schedule (e.g., 3 AM–11 AM), individuals with DSPS get normal, good-quality sleep. But adhering to typical hours (e.g., 11 PM–7 AM) leads to chronic misalignment, causing insufficient sleep and daytime impairment. Effects include:
-
Morning grogginess and prolonged drowsiness
-
Cognitive issues: poor concentration, memory, performance
-
Mood problems: irritability, low motivation, anxiety/depression
-
Bidirectional link: mental health issues can worsen DSPS and vice versa
-
Long-term health risks: disrupted metabolism, weight gain, hormone imbalance
Teens with DSPS may be mislabeled as lazy or unmotivated, but the issue lies in a shifted biological clock. Recognizing such late-sleep patterns helps plan routines that fit.
Symptoms and Causes
Symptoms:
Delayed Sleep Phase Syndrome has distinct symptoms that reflect its core feature—a persistent delay in the timing of sleep. Key signs and symptoms include:
-
Delayed sleep onset:
People with DSPS struggle to fall asleep at conventional times (e.g., 10–11 PM), often not falling asleep until 2–6 hours later. Even when exhausted, they cannot sleep until their internal clock allows it. -
Difficulty waking up:
Because of late sleep onset, waking for morning obligations is extremely difficult. Individuals may sleep through alarms, feel confused or irritable upon waking, and experience severe daytime sleepiness, especially in the morning. This affects concentration, memory, and motivation. Mood issues like irritability or low mood are common. -
Normal sleep on self-set schedules:
When allowed to follow their natural rhythm (e.g., 3 AM–11 AM), people with DSPS usually get normal sleep duration and quality. Sleep efficiency remains high under these conditions. However, when forced to adopt earlier schedules, they lie awake at night and cut sleep short, leading to sleep debt and dysfunction—especially in teens who need 8–10 hours per night. -
Persistent circadian delay:
DSPS is marked by a consistently delayed sleep-wake cycle. Sleep diaries or actigraphy typically show a stable late pattern that shifts even later without constraints. Unlike occasional insomnia, the biological clock is fixed to a late phase. Attempts to shift earlier often fail. Many with DSPS develop coping mechanisms like daytime naps or using caffeine/stimulants, which can further disrupt sleep patterns.
Causes:
Delayed Sleep Phase Syndrome arises from a misalignment between the internal circadian clock and the external environment. In DSPS, the internal clock runs “late” relative to societal time cues (like standard school/work hours). Several factors contribute to this misalignment:
-
Circadian misalignment:
DSPS occurs when the internal circadian clock runs “late” relative to external time cues like school or work hours. Many individuals have an intrinsic circadian period longer than 24 hours (~24.15 h), requiring daily resetting by morning light. If the brain's SCN (suprachiasmatic nucleus) doesn't advance the clock enough, sleep onset drifts later. In DSPS, circadian markers such as core body temperature and melatonin are also delayed, shifting the natural sleep window to the early morning. The “phase angle” between circadian rhythms and sleep may be abnormal, compounding the issue. -
Genetic predisposition:
DSPS often runs in families. Variants in clock genes like CRY1, PER3, and CLOCK contribute to delayed sleep cycles. For instance, a mutation in CRY1 slows circadian cycling. While not always sufficient alone, these genetic factors make individuals more likely to adopt a late sleep pattern. -
Light exposure and lifestyle factors: Evening exposure to blue light (from screens, LEDs, etc.) suppresses melatonin and delays the clock. Research on the Phase Response Curve (PRC) shows that light exposure late at night pushes circadian timing later. Teens who use electronics at night or have irregular schedules (e.g., sleeping in on weekends) experience social jetlag, disrupting circadian stability. Other behavioral contributors include caffeine use, stimulating activities before bed, and poor wind-down routines—all of which exacerbate circadian delay.
-
Altered sleep drive:
DSPS may also involve a slower buildup of homeostatic sleep pressure, especially in adolescents. Napping or oversleeping on weekends reduces this drive, making it harder to fall asleep early. The interaction between a weak homeostatic signal and a circadian rhythm promoting alertness at night delays sleep onset further. -
Insufficient morning reset signals:
Many with DSPS miss out on bright morning light, which is essential for advancing the clock. Dim indoor lighting, late wake times, or overcast climates reduce the effectiveness of this signal. Skipping early-day activities like exercise or breakfast also weakens circadian cues, leaving the clock poorly synchronized.
DSPS stems from a combination of delayed circadian timing, genetic vulnerability, and lifestyle habits that amplify the delay. The clock works—but it’s set to the wrong time. Treatment focuses on advancing the clock and stabilizing it through targeted light exposure, behavioral changes, and routine.
Traditional Treatment Approaches
Managing DSPS often requires a combination of lifestyle changes and strategic interventions to shift the timing of sleep. Traditionally, clinicians and sleep specialists recommend several approaches:
Behavioral strategies & sleep hygiene:
First-line treatment involves improving sleep hygiene and adopting a consistent routine. This includes setting a fixed wake-up time, avoiding naps, and reducing evening light exposure (e.g., limiting screen use, using blue-light filters, and dimming lights). Relaxation techniques in the evening help cue the body for sleep.
Behavioral therapies like chronotherapy gradually shift bedtime later each day to cycle around the clock, aiming to land on an earlier desired bedtime. Though effective, it requires strict discipline and can disrupt daily life. A milder method—phase advance therapy—moves bedtime earlier by ~15 minutes per day and can be effective when paired with other strategies.
Melatonin supplementation:
Low-dose melatonin (1–3 hours before desired bedtime) can cue the body for earlier sleep and shift the circadian phase. It’s widely used in teens and often paired with morning light therapy. While melatonin is generally safe short-term, its timing is critical—taken too late or in high doses, it can cause grogginess or be ineffective.
Bright light therapy (morning exposure):
Morning light therapy is a cornerstone DSPS treatment. Exposure to 10,000 lux from a light box for 60–120 minutes shortly after waking can advance the circadian phase. Ideally, timing is based on biological markers like DLMO (dim-light melatonin onset) or core body temperature minimum, but in practice, light is typically given as early as feasible. Light therapy improves both sleep timing and morning alertness.
Limitations of light therapy:
Conventional light boxes pose challenges for adolescents. Adherence is low due to the early, stationary sessions, bulky devices, and glare. Missed sessions delay progress, and poor timing (e.g., light exposure too late in the morning or at night) may worsen circadian misalignment. Accurate phase timing often requires lab testing, which is impractical in many cases. Side effects like eye strain or agitation can occur. For youth, the rigidity and inconvenience often limit success, prompting interest in wearable light therapy devices as more practical alternatives.
Other interventions:
-
Some medications may help initiate earlier sleep but don’t fix circadian delay.
-
Stimulants or wake-promoting agents can aid alertness but only address symptoms.
-
CBT is being explored to address co-occurring anxiety or learned helplessness.
-
Diagnostic steps should rule out depression, substance use, or other sleep disorders. Polysomnography is usually unnecessary unless another disorder is suspected.
Overall, traditional approaches to DSPS focus on aligning the circadian clock with desired sleep times via lifestyle discipline, appropriately timed light therapy, and sometimes pharmacological aids like melatonin. These can be effective, but in practice many teens struggle to follow through. This is where new innovations, like wearable light devices, are making a difference by offering a more convenient way to get morning light.
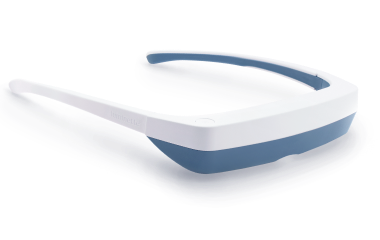
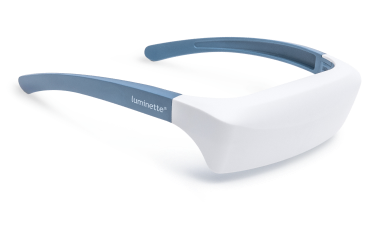
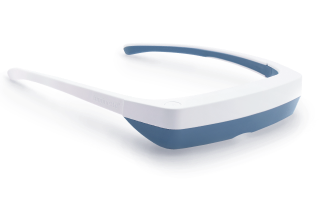
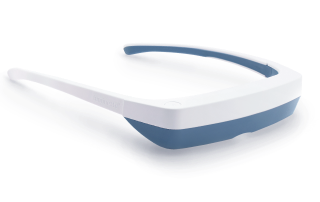
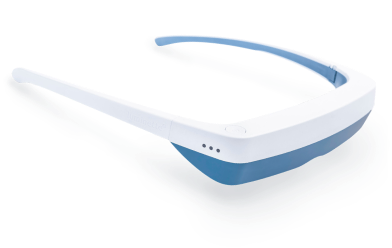
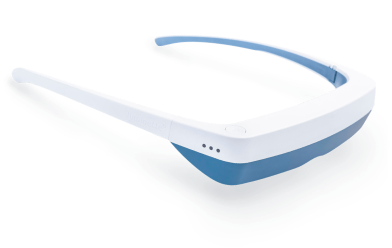
Light Therapy by Luminette: Device Overview
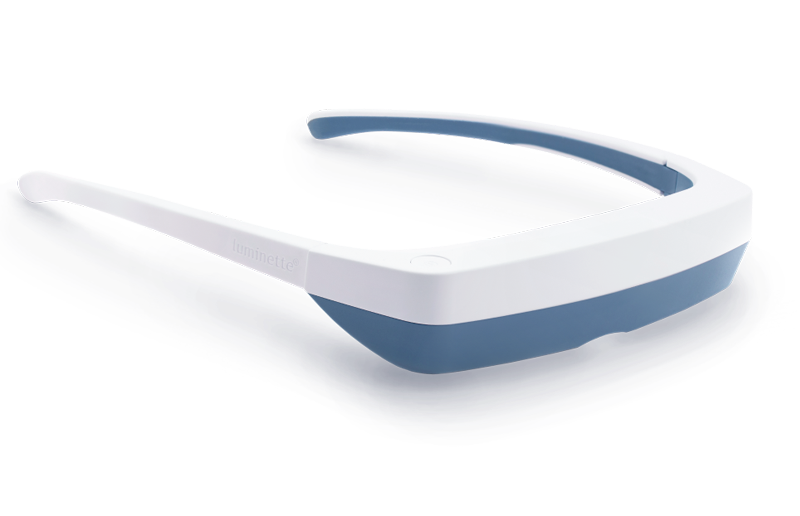
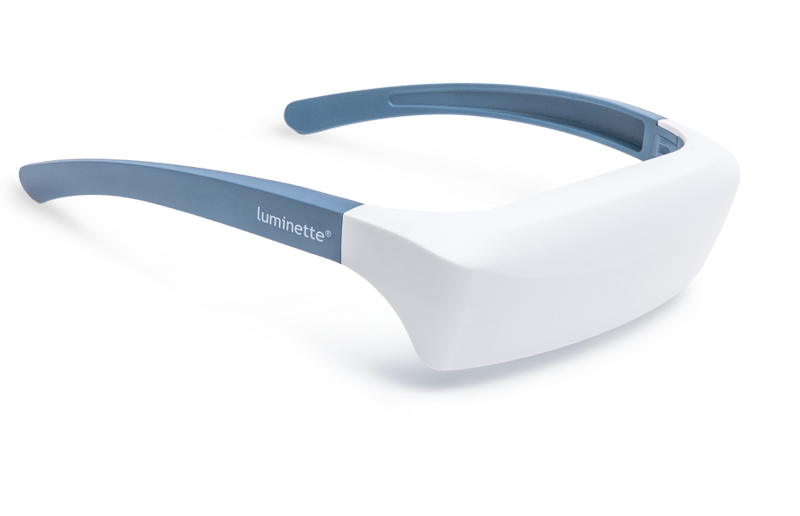
One promising innovation in delivering light therapy for DSPS is the Luminette light therapy device. The Luminette is essentially a pair of wearable light therapy glasses (visor-like goggles) that allow the user to receive bright light while going about their normal morning routine. Unlike a traditional lightboxthat requires sitting in one place, the Luminette is designed to be worn, freeing the user to move around—you could be eating breakfast, reading, or even doing light chores while wearing it. This addresses the key adherence issue by integrating therapy into daily activities.
How does the Luminette work?
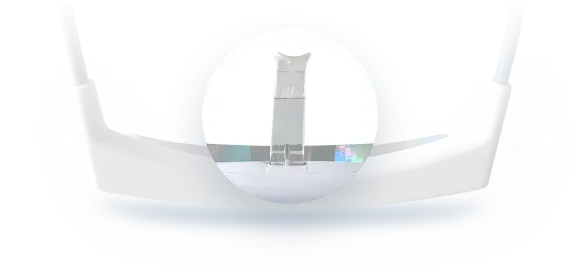
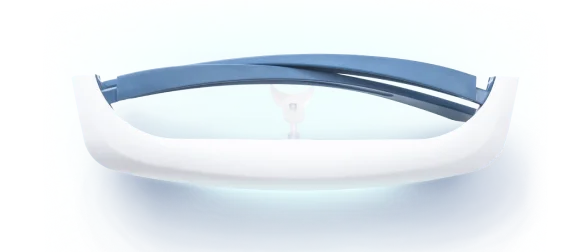
The device uses a series of miniature LEDs to emit a safe, diffused bright white light of about 2,000 lux intensity. The LEDs are positioned along the upper edge of the visor. Through a special optical design (diffractive lenses), the light is directed downward into the eyes at an optimal angle, targeting the lower part of the retina. This part of the retina is rich in photoreceptors that communicate with the circadian clock (via the retinohypothalamic tract to the SCN). By shining light from above (but outside the user’s direct line of sight), the Luminette stimulates the necessary retinal cells without obscuring vision—the user can see their environment normally. Essentially, it tricks the brain into thinking it’s getting morning sunlight. The wavelength of the Luminette’s light is broad-spectrum white, containing the blue wavelengths known to be most effective at suppressing melatonin and shifting the clock (the device specifically has blue-enriched white LEDs, but the blue light is diffused in a comfortable way). At 2,000 lux, it’s not as intense as some larger light boxes (which might be 10,000 lux), but because it’s delivered close to the eyes and at a consistent angle, it provides an effective stimulus within a shorter usage time.
Why is this device more adolescent-friendly?
First, it significantly cuts down the time and compliance burden. Typical use of the Luminette is about 20 to 45 minutes in the morning, which can be done while doing other tasks (versus sitting idle for 90 minutes). For a teenager rushing to school, being able to wear a device during breakfast or while packing their bag is much more feasible. Second, it maintains peripheral vision and mobility—you’re not staring into a blinding lamp, so it feels more natural. The design also eliminates concerns like incorrect positioning; the diffractive lens ensures the light reaches the eyes regardless of head position. In short, the wearable format helps integrate light therapy into a normal routine, potentially improving treatment adherence significantly.
Moreover, the Luminette has been engineered with safety in mind. The intensity (2,000 lux) is sufficient to affect the circadian system but low enough to minimize glare and side effects. The light is UV-free, and the device is lightweight. Because it emits a diffuse glow rather than a direct beam, users usually don’t experience significant eye strain. It also allows the user to maintain sleep hygiene practices—for example, they can still avoid screen time if needed, because the device itself provides the necessary morning light stimulus.
Overall, the Luminette and similar head-mounted light therapy devices represent a modern evolution in chronotherapy tools. They deliver the same type of circadian-resetting light cues as traditional lamps, but in a more practical and user-friendly manner. The convenience of such devices is particularly relevant for adolescents with DSPS, who might otherwise not stick with light therapy at all. But does it actually work as well as the conventional treatment? The next section discusses a pilot study that tested the Luminette in teens with DSPS.
Measurement Tools & Protocol
Throughout the 3-week intervention, the researchers collected data on sleep patterns and daytime alertness using two main self-report instruments, along with daily logs:
Teen Sleep Diary (TSD)
Participants completed the Teen Sleep Diary, developed by the National Sleep Foundation, each morning upon waking for a total of 22 consecutive days, including weekends.
Teens recorded:
-
Bedtime (when they went to bed)
-
Sleep onset (when they actually fell asleep)
-
Wake time (when they got up in the morning)
-
Sleep quality rating (on a 1–5 Likert scale, where 1 = "very poor sleep" and 5 = "excellent sleep")
This diary offered a daily, subjective measure of sleep quality and timing, enabling tracking of trends over the three weeks. Researchers paid special attention to weekday data (Sunday night to Friday morning) for analysis, excluding weekends due to variability in adolescent sleep schedules. This helped isolate effects of the morning light intervention from unrelated fluctuations caused by “social jetlag.”
Pediatric Daytime Sleepiness Scale (PDSS)
The PDSS is a validated 8-item questionnaire designed to measure daytime drowsiness in adolescents. Items include:
-
“How often do you fall asleep or get drowsy during class?”
-
“Are you usually alert during the day?”
Each item is scored on a 0–4 scale, with total scores ranging from 0 to 32. Higher scores indicate more severe daytime sleepiness.
-
The PDSS was administered twice:
-
Day 1 (baseline)
-
End of Week 3 (post-intervention)
-
The PDSS has demonstrated solid internal consistency (Cronbach’s alpha ≈ 0.78) and is widely accepted in adolescent sleep research as a reliable tool.
No Use of Actigraphy or Physiological Phase Markers
Notably, the study did not include actigraphy (wrist-based movement tracking) or objective circadian phase assessments like salivary melatonin (DLMO) or core body temperature minimum. While these biomarkers are often used in circadian rhythm studies to define internal timing, the researchers explained that the exploratory and minimally invasive nature of this pilot justified their exclusion. This approach aligned with guidance from the American Academy of Sleep Medicine, which allows for clinical DSPS studies to rely on behavioral data alone.
However, the authors acknowledged this as a limitation, since the timing of morning light therapy was based on self-reported wake times rather than biological phase markers. All participants were instructed to avoid additional light sources at night, including screens after 9 PM, to reduce external influences on circadian timing.
Protocol and Intervention Design
The study began with a baseline day (Day 1):
-
Teens completed the PDSS
-
Sleep diaries were initiated
-
No treatment was administered
From Day 2 onward, participants received either the Luminette device or a placebo device each morning for 45 minutes. They recorded their use of the device daily in their sleep diary, including any difficulties or noncompliance.
To control for variability, the researchers excluded data from Friday and Saturday nights in their primary weekly analyses, focusing instead on five consecutive weekday nights per treatment week (Week 1, Week 2, Week 3).
Data Analysis Strategy
Given the small sample size (5 teens per group), the study relied on non-parametric statistical methods:
-
For weekly sleep data (e.g., average sleep onset, duration, and subjective sleep quality), researchers used the Mann–Whitney U test, which is robust for small samples and ordinal data.
-
For PDSS scores, they employed a two-way repeated-measures ANOVA with two factors:
-
Group (Luminette vs. placebo)
-
Time (pre-treatment vs. post-treatment)
-
This design allowed them to examine both overall effects and interaction effects (i.e., whether the change in sleepiness differed between groups).
-
Statistical significance was set at p < 0.05.
Despite the absence of objective physiological markers, the study applied well-established subjective tools suitable for adolescent populations and early-phase research. By collecting daily sleep logs and standardized alertness scores, researchers were able to monitor both sleep pattern shifts and daytime functioning. The protocol design—including exclusion of weekend data, clear intervention timing, and multiple week-to-week comparisons—enabled a focused evaluation of light therapy's effectiveness in a real-world adolescent context.
Key Findings: Effects of Luminette Light Therapy
After three weeks of intervention, clear and statistically significant differences emerged between the Luminette (experimental) and placebo groups, based on both sleep diaries and daytime sleepiness questionnaires. The results addressed all three core hypotheses (H1–H3).
1. Sleep Onset Timing & Sleep Duration (H1)
-
Week 1: No significant differences emerged initially. In fact, the Luminette group showed a slight delay in sleep onset compared to placebo (p ≈ 0.056), likely reflecting an adjustment period to morning light exposure.
-
Week 2: Marked improvements appeared. The Luminette group experienced a significantly earlier sleep onset (Mann–Whitney U test: Z = –2.627, p = 0.008) and a notable increase in total sleep time (Z = –2.312, p = 0.016).
-
Week 3: These improvements were sustained or enhanced. Sleep onset remained significantly earlier (p = 0.008), and nightly sleep duration stayed longer (p = 0.008) than in the placebo group.
Magnitude: By Week 2, Luminette users were falling asleep 1–2 hours earlier than baseline and compared to the placebo group. This advanced their average sleep onset to around midnight, versus 2–3 AM in controls. With earlier sleep, they gained ~1 additional hour of sleep per school night.
Conclusion: These findings support H1—wearable morning light therapy produced a meaningful circadian phase advance, helping teens align sleep with school schedules and reducing weekday sleep deprivation.
2. Subjective Sleep Quality (H2)
-
Week 1: Interestingly, the placebo group reported better sleep quality early on (mean ≈ 2.4 vs. 2.0 in Luminette users, p = 0.016). This may reflect initial overstimulation from light exposure or a placebo effect.
-
Weeks 2 & 3: The Luminette group’s ratings improved significantly. By Week 2, they reported an average quality
-
Statistical support: Both Week 2 and Week 3 differences were statistically significant (p = 0.008).
Interpretation: As their circadian phase adjusted and sleep duration increased, Luminette users began to perceive their sleep as more restorative. The improved timing, duration, and alignment with biological night likely drove this shift.
Conclusion: These results confirm H2—morning light therapy improved subjective sleep quality, especially after the initial adaptation period.
3. Daytime Sleepiness (H3)
-
Baseline (Day 1): Both groups had similarly high PDSS scores (~23/32), reflecting clinically significant daytime drowsiness.
-
Post-treatment (Week 3):
-
Luminette group: PDSS scores dropped to ~14.8, indicating moderate sleepiness only.
-
Placebo group: Scores remained essentially unchanged (~22).
-
Statistical analysis:
-
Two-way repeated-measures ANOVA revealed:
-
Significant Time × Group Interaction (p < 0.001 )
-
Main effects of Time and Group
-
Effect size: Partial η² ≈ 0.90 (very large)
-
Practical implication: Teens in the Luminette group felt more alert, stayed awake in class, and likely showed better mood and cognitive engagement. In contrast, the placebo group’s unaltered sleep schedule led to persistent daytime fatigue.
Conclusion: Results strongly support H3—light therapy with Luminette significantly reduced daytime sleepiness and enhanced daily functioning.
4. Safety & Tolerability
-
No serious adverse effects were reported.
-
No participant dropouts occurred.
-
Some users may have experienced mild transient effects (e.g., slight headaches, stimulation), especially in Week 1, but these did not interfere with adherence.
Device acceptance: The wearable format improved usability and minimized side effects (e.g., no eye strain or glare, UV-free light). The lack of complaints suggests good tolerability, even in a teen population.
Summary
This pilot trial demonstrated that Luminette wearable light therapy:
-
Advanced sleep timing by 1–2 hours
-
Increased total sleep duration on school nights
-
Improved perceived sleep quality
-
Reduced daytime sleepiness significantly
All findings were statistically robust and clinically meaningful, validating the researchers’ hypotheses (H1, H2, H3). Importantly, the device offers a practical, user-friendly alternative to traditional light boxes, especially for adolescents with DSPS who often struggle with rigid protocols.
While the sample size was small, the strong effects justify larger trials and suggest that wearable light therapy could become a mainstream treatment for circadian sleep disorders in youth.
Discussion & Implications
1. Efficacy Confirmation
This pilot trial offers proof-of-concept that wearable light therapy using the Luminette device can significantly improve outcomes in Delayed Sleep Phase Syndrome (DSPS). All three hypotheses were confirmed:
-
H1: Circadian phase advanced (earlier sleep onset, longer duration).
-
H2: Subjective sleep quality improved.
-
H3: Daytime sleepiness reduced.
The results align with prior findings that morning bright light is a powerful phase-advancing agent. Importantly, this study extends that evidence by showing it can be delivered effectively via wearable light glasses, not just stationary light boxes—a critical innovation for adolescents, who often struggle with adherence to rigid treatments.
2. Practical Advantages of the Luminette Device
The wearable format addressed key compliance issues:
-
Allowed teens to move freely while receiving treatment.
-
Delivered 2,000 lux of blue-enriched white light at a consistent angle close to the eyes.
-
Achieved outcomes comparable to 10,000 lux boxes, likely due to proximity and usage consistency.
Its design, portability, and integration into daily routines likely boosted adherence. For adolescents, this format feels less clinical, more tech-forward, and better fits into modern lifestyles—potentially transforming light therapy adherence rates.
3. Holistic Benefits Beyond Sleep Timing
The intervention delivered more than a schedule adjustment:
-
Improved daytime alertness, which may translate to better school performance, mood, and overall functioning.
-
A non-pharmacological approach appeals to families wanting to avoid medication side effects or dependency.
Light therapy leverages the body’s own physiology—entraining the circadian system naturally through light exposure—offering a safe, effective alternative to melatonin or sedatives.
4. Study Limitations
Sample Size:
-
Only 10 participants (5 per group).
-
Although effects were significant, results must be interpreted with caution due to high variability risk and limited generalizability.
Short Duration:
-
The 3-week treatment period showed initial efficacy but didn't assess long-term sustainability.
-
No follow-up phase to determine if benefits persist after stopping therapy.
-
Weekend sleep data were excluded—important in real-world adolescent life, where weekend habits often undermine weekday improvements.
No Circadian Phase or Chronotype Measures:
-
Lacked data on DLMO, core temperature, or morningness-eveningness preference, which could inform personalization and timing optimization.
-
Future studies should distinguish between biological vs. behavioral DSPS cases.
Self-Reported Outcomes:
-
All data were subjective (sleep diaries, PDSS), introducing potential expectation bias.
-
No actigraphy or polysomnography to validate diary accuracy or detect sleep efficiency/architecture changes.
Placebo Design Considerations:
-
The control device used orange light, which may have had mild circadian effects or influenced user perception.
-
Still, the large between-group differences suggest a real therapeutic effect from the active device.
5. Future Research Directions
Larger, longer trials are needed to:
-
Confirm efficacy in broader populations.
-
Examine the sustainability of phase shifts post-treatment.
-
Evaluate real-world use across weekends and holidays.
-
Explore outcomes in other age groups (preteens, college students, adults) and related conditions (e.g., SAD, shift work disorder, jet lag).
Include objective measures:
-
Actigraphy to track actual sleep/wake behavior.
-
DLMO and core body temperature to quantify internal circadian shifts.
-
Hormonal markers (e.g., cortisol) to assess biological impact.
Compare treatments:
-
Luminette vs. melatonin, traditional light box, or combined protocols.
-
Determine if light therapy can replace or enhance pharmacological interventions.
Assess long-term safety and health outcomes:
-
Monitor for eye health, mood stability, and academic performance.
-
Determine whether therapy improves broader well-being and lifestyle patterns.
6. Clinical Implications
For pediatricians and sleep specialists, wearable light therapy offers a practical, rapid-onset, non-drug treatment for DSPS:
-
Results are visible in 1–2 weeks.
-
Compatible with school routines.
-
May reduce reliance on medications or drastic measures (e.g., shifting school start times).
The study reinforces the importance of:
-
Morning light exposure to advance the circadian clock.
-
Evening light avoidance to support melatonin release.
Even without Luminette access, clinicians can encourage morning daylight exposure and reduced nighttime screen use as foundational circadian strategies.
This pilot study demonstrates that Luminette wearable light therapy can successfully shift circadian timing, improve sleep quality, and enhance daytime functioning in adolescents with DSPS. It offers a convenient, effective, and well-tolerated alternative to traditional light therapy and a promising new tool in circadian medicine. As technology evolves, combining behavioral insights with innovative delivery methods may unlock better sleep health for teens—and beyond.
Conclusions
Delayed Sleep Phase Syndrome (DSPS) in adolescents is a disorder of timing—where the internal circadian rhythm is delayed relative to social demands. This mismatch leads to late bedtimes, morning wake-up difficulties, chronic sleep deprivation, and impaired school performance or mood. While traditional treatments such as sleep hygiene, chronotherapy, melatonin, and bright light therapy can help, adherence—particularly to fixed-location light boxes—has often been poor, limiting effectiveness in the real world.
The emergence of wearable light therapy devices, like the Luminette, represents a modern solution. This pilot study demonstrated that delivering blue-enriched white light through a wearable device each morning produced meaningful benefits:
-
Advanced circadian timing (earlier sleep onset by up to 2 hours),
-
Longer weekday sleep duration, and
-
Significantly reduced daytime sleepiness.
These improvements were achieved without medications or lifestyle overhaul—simply by wearing the device during regular morning routines. The 2,000 lux light intensity, directed efficiently to the lower retina, mimicked clinical-grade therapy in a user-friendly, mobile format ideal for teens.
From a clinical perspective, Luminette therapy offers several advantages:
-
It enables mobility during treatment.
-
Encourages daily use through integration with routine, and
-
Avoids side effects linked to pharmacological approaches.
For clinicians, caregivers, and youth, this means a realistic, accessible method to reset the body clock without significant disruption.
While larger trials are needed to confirm long-term efficacy, these findings suggest that wearable light therapy is ready for clinical integration. As protocols evolve—e.g., refining timing, duration, and combinations with melatonin or light avoidance—the utility of devices like Luminette will likely expand. Just as eyeglasses correct vision, “light glasses” may help recalibrate circadian rhythms .
Ultimately, DSPS management is shifting toward methods that align with the body’s biology. Morning light is the most powerful natural cue for circadian adjustment, and this study shows it can now be delivered conveniently at home. For teens often mislabeled as lazy due to their sleep patterns, wearable light therapy offers a life-changing intervention—restoring alignment, improving alertness, and helping them engage in daily life with confidence.
FAQ
What is Delayed Sleep Phase Syndrome (DSPS)?
DSPS is a circadian rhythm disorder where a person’s internal clock is delayed, causing them to fall asleep and wake up much later than desired. It’s biological, not behavioral, and often results in difficulty waking for school or work despite being able to sleep well at preferred hours.
How common is DSPS among adolescents?
DSPS affects an estimated 7–16% of adolescents, making it relatively common. It often begins during puberty when natural biological shifts and social habits push sleep later.
What treatments exist for DSPS besides light therapy?
Other treatments include melatonin supplements in the evening, strict sleep hygiene routines, chronotherapy (gradually shifting sleep times), and, in some cases, short-term medications or school schedule adjustments. A combination approach is often most effective.
How does Luminette Light Therapy differ from traditional light boxes?
The Luminette is a wearable device that allows users to move freely while receiving morning light therapy, unlike traditional stationary lamps. It delivers effective circadian light at lower intensity by directing it precisely to the eyes, improving convenience and adherence.
What evidence supports the use of Luminette for DSPS?
A 3-week randomized study in teens with DSPS showed that the Luminette significantly advanced sleep onset, increased sleep duration, improved sleep quality, and reduced daytime sleepiness compared to a placebo. These results align with established science on circadian light therapy.
Are there any risks or limitations to Luminette Light Therapy in adolescents?
Luminette is generally safe, but mild side effects like eye strain or headache can occur. Proper timing is crucial—using it too late in the day or inconsistently may reduce effectiveness or worsen sleep timing.









































 Please note
Please note